In the last several years, Tau-directed therapies have gained more interest as a strategy for treating Alzheimer’s Disease.
Contrary to the APP mouse models, Tau mouse models appear to have a higher intrinsic variability in the onset and progression of the pathology, further complicating in-vivo proof-of-concept studies. Against this background, reMYND’s TAU.P301L transgenic mouse model of progressive tau-o-pathy, has proven to be a valuable tool for efficacy testing of such Tau-directed therapies. The model is characterized by a strong increase of hyper-phosphorylated and insoluble Tau in function of age concomitant to an impaired motor function, which strongly correlates in time with the pathology, allowing for a longitudinal follow-up.
In a recent study performed at reMYND, a Tau-directed reference antibody was chronically administered to Tau.P301L mice. The study showed a significant improvement in clasping behaviour.
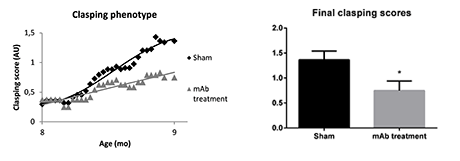
Figure 1: Animals were scored for clasping behaviour using the tail suspension test during the study
In line with the improvement in clasping behaviour, a decrease in insoluble Tau and various phospho-Tau read-outs were measured in biochemical and immunohistochemical assays, while no impact on total Tau in whole brain extract was observed.
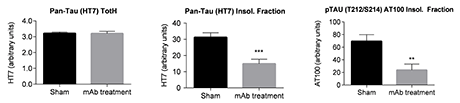
Figure 2: Measurements of pan-Tau (left) in total homogenate, Sarkosyl insoluble fraction (middle) and phospho-Tau in the Sarkosyl insoluble fraction (right) of brain stem from Tau.P301L mice.
Clasping score correlated highly with hyper-phospho-Tau epitopes, with more than 70% of variability explained. These results support an effect of the Tau directed antibody treatment and affirm the Tau.P301L model as a useful tool for efficacy testing of tau-directed therapies.
For more information on reMYND’s transgenic mouse models please visit our website or contact us at cro@remynd.com. You can also contact us at this email address to set-up a meeting at the SfN conference in San Diego from 10-12 November if you want to explore in-person how we could help you.
Kind regards,
The CRO team













