Key characteristics
- Progressive β-amyloid plaque development at a later age (from 10 months) in cortex, hippocampus and subiculum
- Pyroglutamate-modified Aβ42 (Aβ3(pE)-42) is detected in the insoluble brain fraction from 12 months onwards
- Progressive microgliosis and astrocytosis from an age of 10 months
- Cognitive impairment in the Morris water maze paradigm and hippocampal LTP deficit from an age of 6 months
- Early Aβ-induced GSK3-activation and mTAU phosphorylation in hippocampus and dystrophic neurites containing hyperphosphorylated mTau (no tangle-pathology)
- CAA pathology and micro-bleedings from an age of 15-18 and 25-30 months, respectively
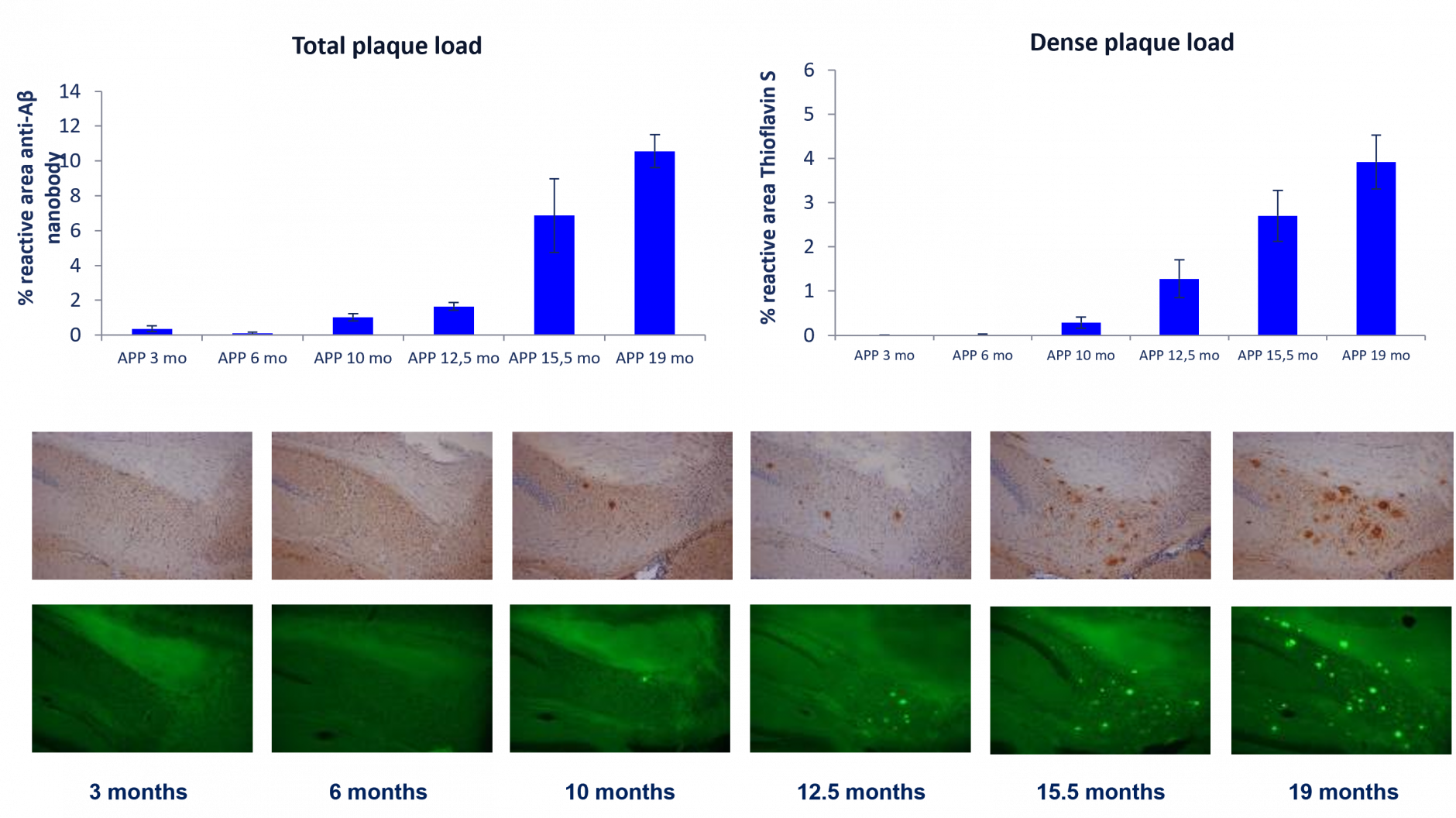
Progressive total plaque load (an anti-Aβ antibody) and dense plaque load (Thioflavin S) in the subiculum as measured by IHC (mean ± SEM, N = 5-9).
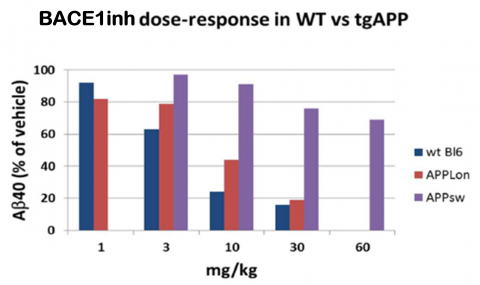
The London mutation [V717] is a preferred model for anti-BACE1 approaches. The Swedish mutation might result in an aberrant subcellular localization of APP (Jacobsen et al., 2014).
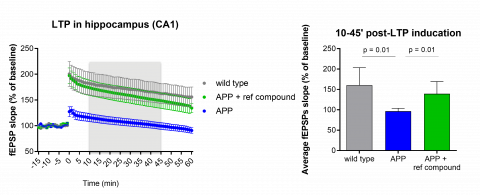
Long term potentiation deficit (induced by a high frequency stimulation) in the CA1 region of the hippocampus in 8 month old APP[V717] animals and age-matched littermates can be restored with a therapeutic compound (mean ± SEM, n = 5 per group).
Interested to see more data?
We have a more extensive datapackage available. Contact us at cro[at]remynd.com to set up a meeting and discuss how we translate your mechanism of action into an effective study design.
Literature
Describing papers:
- Original paper: Moechars et al., 1999: Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. DOI: 10.1074/jbc.274.10.6483
- Tanghe et al., 2010: Pathological Hallmarks, Clinical Parallels, and Value for Drug Testing in Alzheimer's Disease of the APP[V717I] London Transgenic Mouse Model. DOI: 10.4061/2010/417314
- Van Dorpe et al., 2000: Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the london mutant of human APP in neurons. DOI: 10.1016/S0002-9440(10)64644-5
- Terwel et al., 2008: Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. DOI: 10.2353/ajpath.2008.070904
Therapeutic intervention papers:
- Jacobsen et al, 2014: Combined Treatment with a BACE Inhibitor and Anti-Aβ Antibody Gantenerumab Enhances Amyloid Reduction in APPLondon Mice. DOI: 10.1523/JNEUROSCI.1405-14.2014
- Janssens et al., 2021: Passive immunotherapy with a novel antibody against 3pE-modified Aβ demonstrates potential for enhanced efficacy and favorable safety in combination with BACE inhibitor treatment in plaque-depositing mice. DOI: 10.1016/j.nbd.2021.105365













