Key characteristics
- Progressive hyperphosphorylation and conformational change of Tau
- Neurofibrillary tangles are mainly observed in the brain stem, spinal cord and midbrain and to lesser extent in the cerebral cortex
- Astrocytosis, microgliosis and neuronal loss in regions with Tau pathology
- Cognitive impairment in passive avoidance paradigm and LTP deficit from 5 months of age
- Correlation of motoric impairment and survival with Tau hyperphosphorylation
- Correlation of motoric impairment with spinal cord pathology
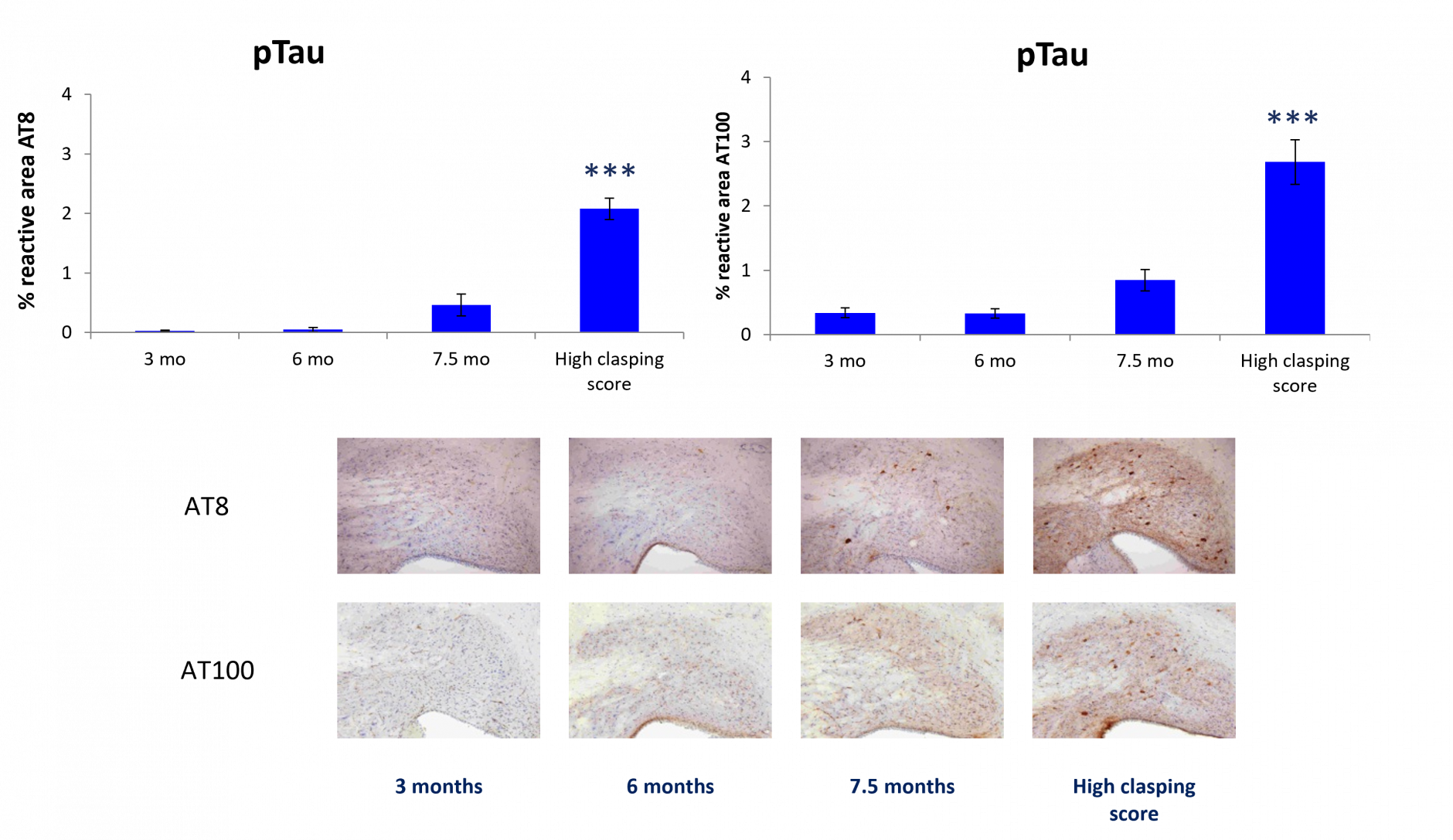
Age dependent increase of Tau pathology (AT8 = pSer202 pThr205 Tau, AT100 = pThr212/pSer214 Tau) in the interposed cerebellar nulceis, anterior and posterior part (LAT/Int/AP) as measured by IHC (mean ± SEM, n = 15). Statistics: One-way ANOVA
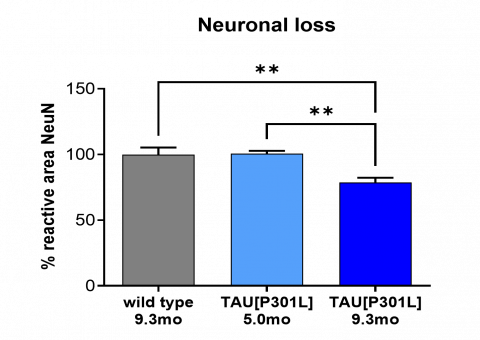
Progressive neuronal loss (NeuN) in the subthalamic nucleus and zona inserta (STH/ZI, n = 14, 15 and 28 respectively), as measured by IHC. Statistics: Two-way ANOVA, Tukey’s multiple comparison
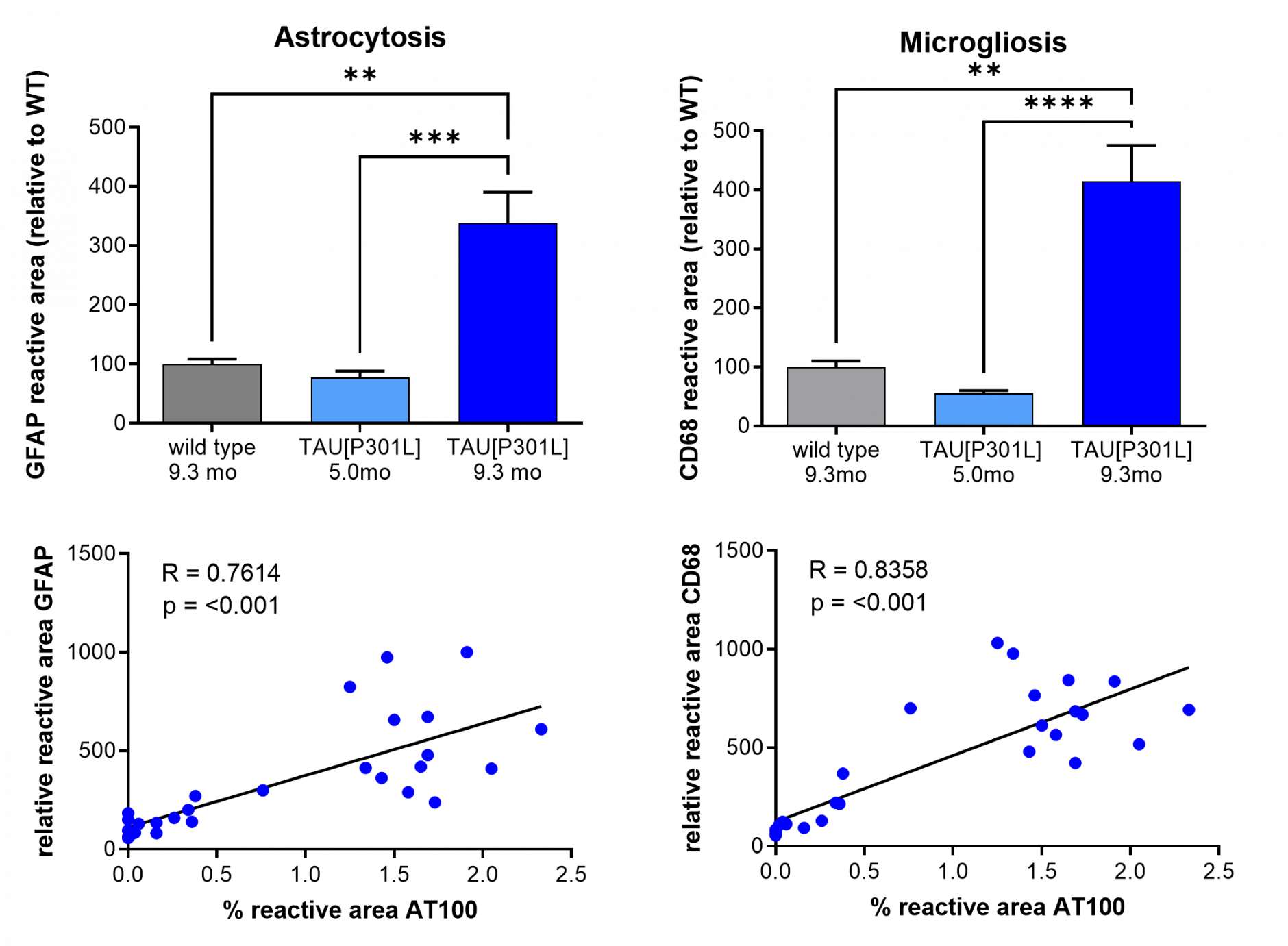
Progressive astrocytosis (GFAP) and microgliosis correlates with Tau pathology in the interposed cerebellar nulceis, anterior and posterior part (LAT/Int/AP) as measured by IHC (mean ± SEM, n = 14, 15 and 28 respectively). The correlation graphs are only for 9.3 months of age. Statistics: Two-Way ANOVA, Tukey’s multiple comparison
Interested to see more data?
We have a more extensive datapackage available. Contact us at cro[at]remynd.com to set up a meeting and discuss how we translate your mechanism of action into an effective study design.
Literature
Describing papers:
- Original paper: Terwel et al., 2005: Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. DOI: 10.1074/jbc.M409876200
- Chong et al., 2011: Synaptic dysfunction in hippocampus of transgenic mouse models of Alzheimer’s disease: A multi-electrode array study. DOI: 10.1016/j.nbd.2011.07.006
Therapeutic intervention papers:
- Bright et al. 2015: Human secreted tau increases amyloid-beta production. DOI: 10.1016/j.neurobiolaging.2014.09.007
- Hansen et al. 2016: The GLP-1 receptor agonist liraglutide reduces pathology-specific tau phosphorylation and improves motor function in a transgenic hTauP301L mouse model of tauopathy. DOI: 10.1016/j.brainres.2015.12.052
- Theunis et al. 2013: Efficacy and Safety of A Liposome-Based Vaccine against Protein Tau, Assessed in Tau.P301L Mice That Model Tauopathy. DOI: 10.1371/journal.pone.0072301
- Pre-clinical data is listed in the iPierian patent WO 2014/028777













